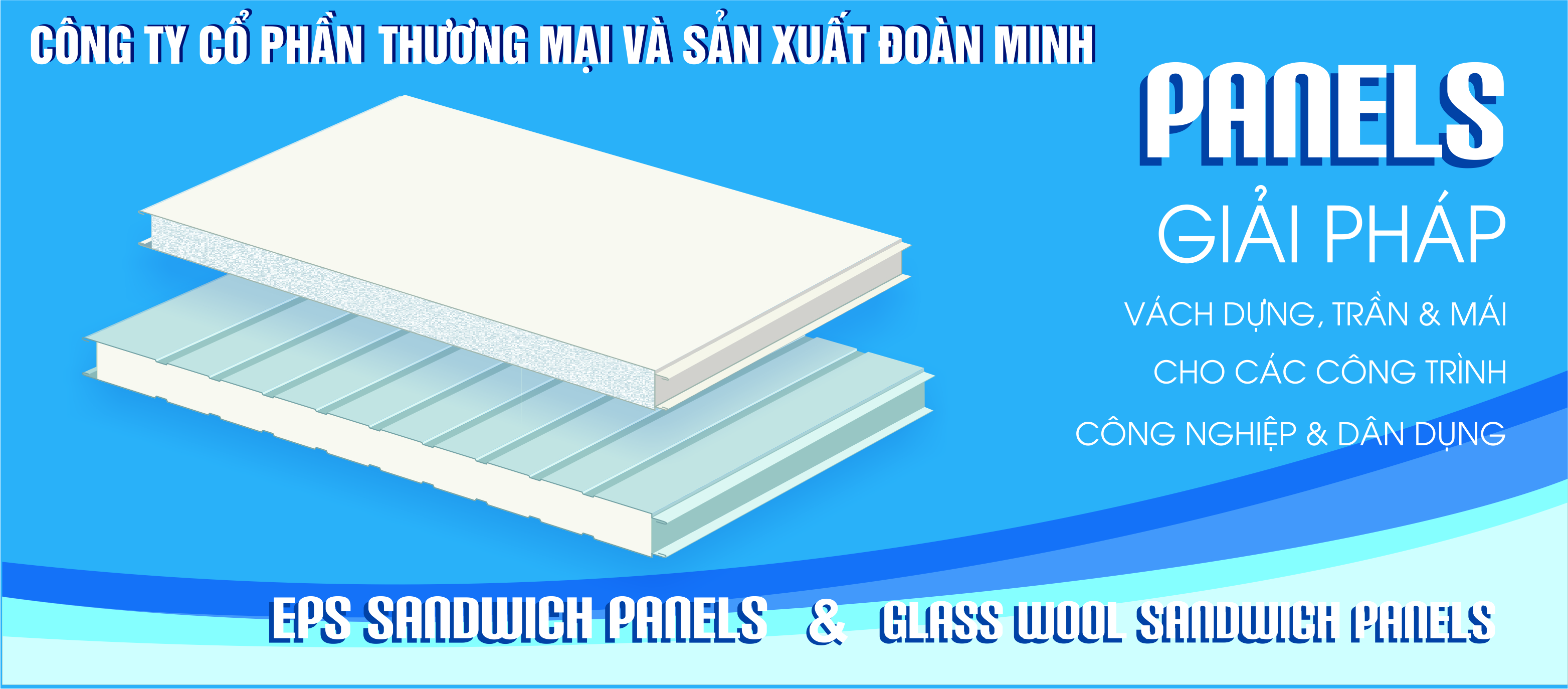
Giơi thiệu chung
Trải qua nhiều năm xây dựng và trưởng thành, hiện nay, công ty CPTM&SX Đoàn Minh đã trở thành một doanh nghiệp lớn ở khu vực phía Bắc, cung cấp đầy đủ các biên dạng sóng đáp ứng mọi nhu cầu của người sử dụng. Sản phẩm tôn lợp mạ màu của công ty Đoàn Minh luôn được người tiêu dùng và các chủ đầu tư tin tưởng. Đoàn Minh luôn đảm bảo cam kết về chất lượng hàng hóa, chất lượng dịch vụ, nhằm đáp ứng yêu cầu ngày càng cao của khách hàng.